Quality and Certifications
At Galena Pharma, we are dedicated to produce safe, high-quality, and effective health products. Our highest quality standards span the entire product lifecycle from development and registration to manufacturing and lifecycle management. The purpose of our quality commitment is to ensure that our clients receive comprehensive support tailored by their needs and that the products we manufacture meet the expectations of the end-users.
Quality as an organisational culture
At the heart of Galena Pharma are dedicated and skilled people who, in their daily work, ensure the delivery of quality products and our commitment to compliance and customer trust. Our people are the foundation of our success, and their expertise and proactive mindset ensure that we consistently deliver safe and high-quality end products. We foster a culture where every employee takes ownership of continuous improvement, actively identifying opportunities to enhance processes, reduce risks, and ensure compliance.
Our organizational culture is founded on trust, transparency and innovation, which allows us to respond quickly to customer needs while maintaining the highest standards of quality and compliance. We believe that by building an open culture of trust, we strengthen our ability to be a reliable and agile partner in the healthcare industry.
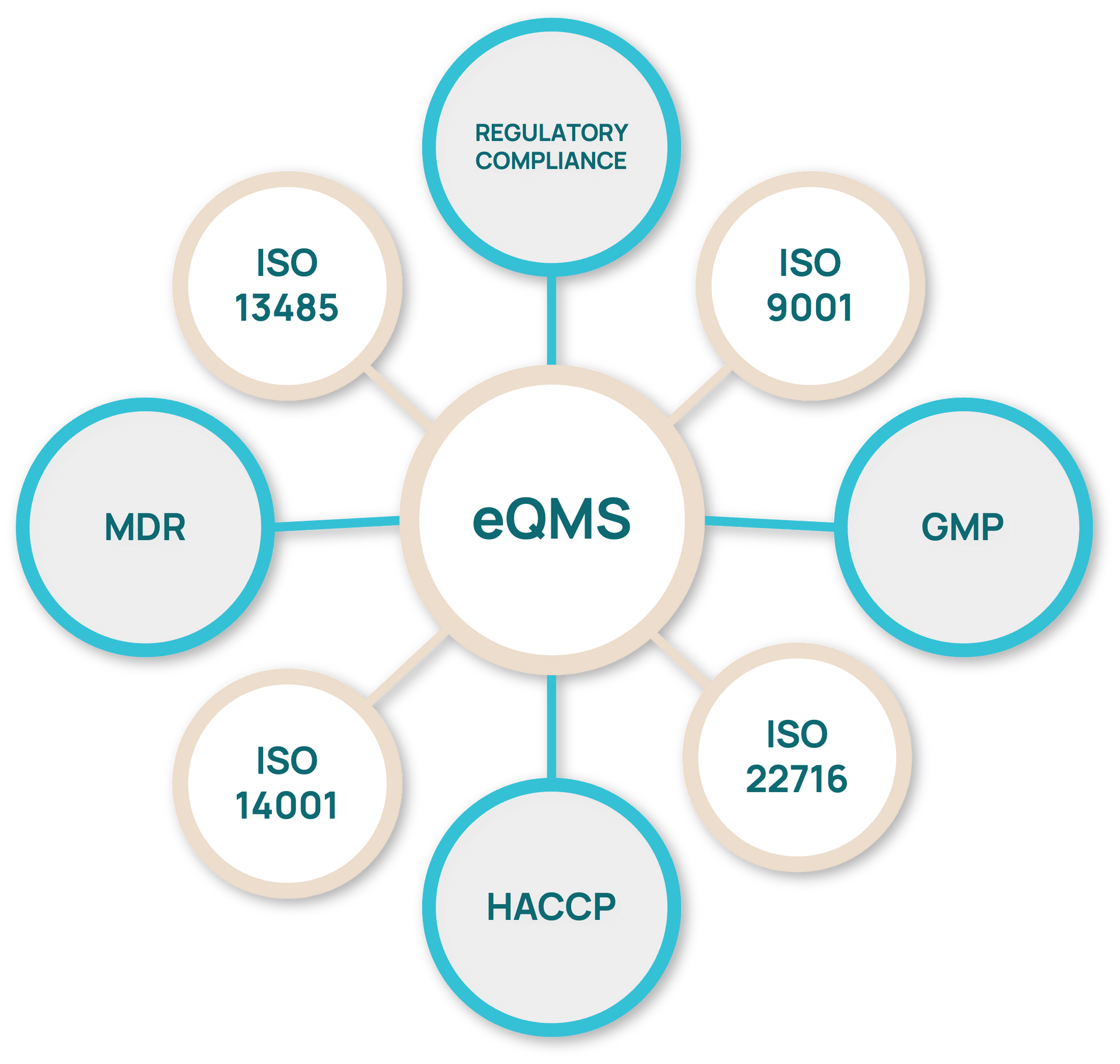
eQuality Management System
The quality of our products comes from our people, and their operations are guided by our electronic quality system.
All our processes are guided by the EU GMP guidelines and adhere to internationally recognized standards, including: ISO 9001:2015, ISO 13485:2016, ISO 14001:2015, ISO 22716:2007, ISO 45001:2023, ISO 27001:2022.
We fully comply with the EU Medical Device Regulation (MDR 2017/745). As a legal manufacturer under MDR, Galena Pharma ensures that all activities, procedures, and documentation meet the highest regulatory standards.
Certifications and Permits
Industrial manufacture and packaging of pharmaceuticals
Cosmetics
Environmental management system
Quality management system
Continuous improvement for sustainable future
At Galena, we acknowledge the significant environmental and social challenges facing the world today.
Sustainability is integral part of our quality management system and since 2022 we have been ISO 14001 certified company. In 2025 we published our first Sustainability Report which complies with the ESG standards. We have identified the most important areas of action in our operations and are implementing measures according to the set KPIs. Galena Pharma is committed to transparency, sustainability, and long-term value creation for all stakeholders.
Quality products from the beginning to the end
Everything we do is based on quality. With years of experience and a dedicated team of professionals, we ensure that the products we manufacture meet the highest quality standards in the industry. If you want to know more about our quality principles, don’t hesitate to contact us!




